Mains > Social justice > Health > Pharmaceutical sector
SYLLABUS
GS 2 >> Public Health >> Regulation of pharmaceuticals
REFERENCE NEWS
Recently, several Indian newspapers have come under scrutiny for publishing misleading advertisements about unapproved miracle drugs. A prominent example is an advertisement for a so-called miracle injection for weight loss that contains Semaglutide. Even though many of these miracle drugs do not have regulatory approval for sale in India, they are still being administered by some doctors to well-off patients.
ABOUT MIRACLE DRUGS
Miracle drugs refer to pharmaceutical agents that revolutionize the management of specific health conditions due to their outstanding effectiveness, innovative therapeutic benefits, and their significant enhancement of patient health.
Examples include:
In India, however, there are instances of the use of miracle drugs that have not received official approval. Examples include Semaglutide and Fen-Phen for weight loss, and Adcetris for the treatment of blood cancers.
DRUG APPROVAL PROCESS IN INDIA
The regulatory framework for drug approval in India is governed by the Drugs and Cosmetics Act, 1940, and the Drugs and Cosmetics Rules, 1945.
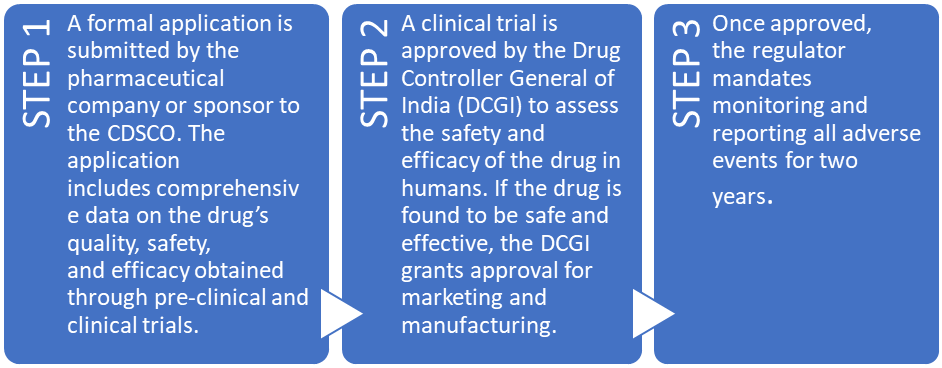
LEGAL FRAMEWORK OF DRUG REGULATION IN INDIA 1.The Drugs and Cosmetics Act, 1940: This is the primary legislation for the regulation of drugs and cosmetics in India. It controls the import, manufacture, distribution, and sale of drugs and cosmetics to ensure they are safe, effective, and meet quality standards. The act is supplemented by the Drugs and Cosmetics Rules, 1945, which provide detailed guidelines on standards, licensing, and the regulatory framework for drug control. 2.The Pharmacy Act, 1948: This act regulates the profession of pharmacy in India. It establishes the Pharmacy Council of India and the State Pharmacy Councils, which are responsible for the registration of pharmacists and the regulation of professional practices. The act aims to ensure that only qualified individuals dispense drugs in India. 3.The Drug and Magic Remedies (Objectionable Advertisements) Act, 1954: This act controls the advertisement of drugs in certain cases and prohibits the advertisement of remedies as magic solutions for certain diseases and disorders. It aims to protect consumers from misleading advertisements related to drug and health remedies. 4.The Narcotic Drugs and Psychotropic Substances Act, 1985 (NDPS Act): While primarily aimed at controlling narcotic drugs and psychotropic substances, this act also impacts the medical use of certain controlled substances. It regulates the cultivation, production, distribution, sale, and use of narcotics and psychotropic substances, ensuring that they are available only for medical and scientific purposes under strict regulatory control. 5.The Drugs (Prices Control) Order, issued under the Essential Commodities Act: This order regulates the prices of drugs in India to ensure that essential drugs are available at reasonable prices. The National Pharmaceutical Pricing Authority (NPPA) is responsible for implementing and enforcing the order, which covers the pricing of drugs and pharmaceuticals. |
However, many Global pharma companies sometimes choose to stay out of the Indian market and not launch drugs in India. In such circumstances, patients can get a licence from the drug regulator based on a doctor’s prescription to import these drugs for personal use. Similarly, hospitals also apply for import licences of these drugs.
IMPACTS OF THE UNAPPROVED MIRACLE DRUGS IN INDIA
WAY FORWARD
CENTRAL DRUGS STANDARD CONTROL ORGANIZATION The Central Drugs Standard Control Organization (CDSCO) is a national regulatory body that is responsible for the safety, efficacy, and quality of drugs, cosmetics, and medical devices in India. The CDSCO's vision is to protect and promote public health.
|
PRACTICE QUESTION
Q: Evaluate the challenges and implications of unapproved miracle drugs on public health and regulatory frameworks in India. (15M, 250W)